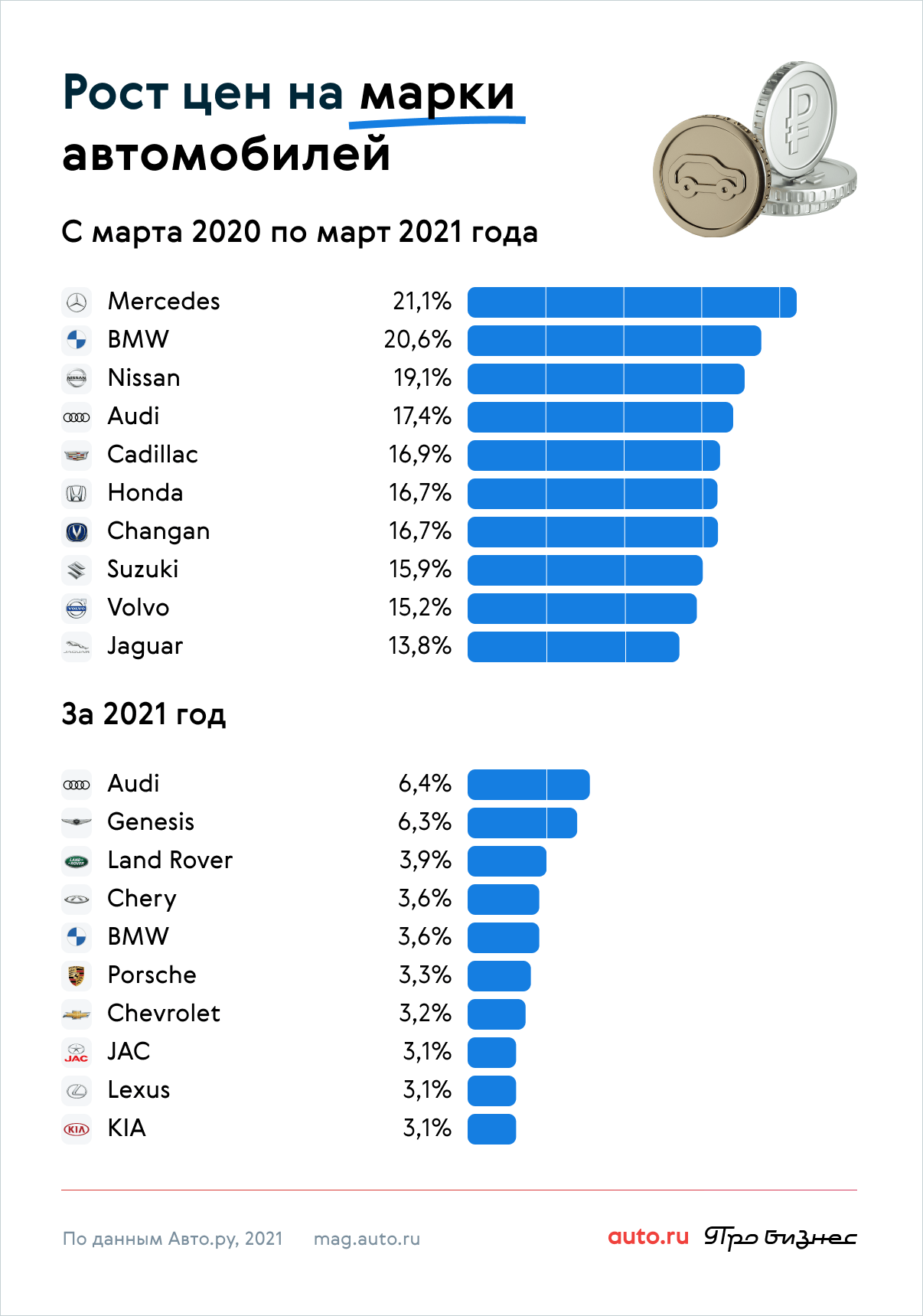
iav dej
Cov iav kua yog ib qho kev daws teeb meem ntawm sodium metasilicate Na2SiO3 (potassium ntsev kuj siv). Nws yog tsim los ntawm dissolving silica (xws li xuab zeb) hauv cov tshuaj sodium hydroxide:
iav dej Qhov tseeb, nws yog kev sib xyaw ntawm cov ntsev ntawm ntau yam silicic acids nrog ntau qib ntawm polymerization. Nws yog siv los ua impregnation (piv txwv li, los tiv thaiv phab ntsa los ntawm noo noo, tiv thaiv hluav taws), ib feem ntawm fillers thiab sealants, rau zus tau tej cov ntaub ntawv silicone, raws li zoo raws li ib tug zaub mov additives los tiv thaiv caking (E 550). Kev lag luam muaj kua iav tuaj yeem siv tau rau ntau qhov kev sim zoo nkauj (vim tias nws yog tuab, kua dej, nws yog siv los ntawm diluting nws nrog dej hauv qhov sib piv ntawm 1: 1).
Nyob rau hauv thawj qhov kev sim, peb yuav precipitate ib tug sib tov ntawm silicic acids. Rau kev sim, peb yuav siv cov kev daws teeb meem hauv qab no: kua iav thiab ammonium chloride NH.4Cl thiab daim ntawv qhia kom kuaj cov tshuaj tiv thaiv (duab 1).
Chemistry - ib feem ntawm kua iav 1 - MT
Cov iav ua kua ua ntsev ntawm cov kua qaub tsis muaj zog thiab lub hauv paus muaj zog hauv cov kua dej yog hydrolyzed ntau thiab yog alkaline (duab 2). Ncuav cov tshuaj ammonium chloride (daim duab 3) rau hauv lub beaker nrog dej iav tov thiab do cov ntsiab lus (duab 4). Tom qab qee lub sij hawm, gelatinous loj yog tsim (daim duab 5), uas yog sib xyaw ntawm silicic acids:
(tiag SiO2?nGn2O? silicic acids nrog ntau qib ntawm hydration yog tsim).
Lub beaker cov tshuaj tiv thaiv mechanism uas sawv cev los ntawm cov ntsiab lus sib npaug saum toj no yog raws li hauv qab no:
a) sodium metasilicate nyob rau hauv cov tshuaj dissociates thiab undergoes hydrolysis:
b) ammonium ions teb nrog hydroxide ions:
Raws li hydroxyl ions tau noj hauv cov tshuaj tiv thaiv b), qhov sib npaug ntawm cov tshuaj tiv thaiv a) hloov mus rau sab xis thiab, vim li ntawd, silicic acids precipitate.
Hauv kev sim thib ob, peb loj hlob "cov nroj tsuag tshuaj". Cov kev daws teeb meem hauv qab no yuav xav tau rau kev sim: kua iav thiab hlau ntsev? hlau (III), hlau (II), tooj liab (II), calcium, tin (II), chromium (III), manganese (II).
Chemistry - ib feem ntawm kua iav 2 - MT
Cia peb pib qhov kev sim los ntawm kev qhia ob peb lub pob zeb ntawm cov hlau chloride (III) ntsev FeCl rau hauv lub raj kuaj.3 thiab ib qho kev daws ntawm kua iav (duab 6). Tom qab ib pliag, xim av? (Duab 7, 8, 9), los ntawm insoluble hlau (III) metasilicate:
Tsis tas li ntawd, ntsev ntawm lwm cov hlau tso cai rau koj kom tau txais txiaj ntsig zoo:
- tooj (II)? duab 10
- chromium (III)? duab 11
- hlau (II)? duab 12
- calcium? duab 13
- manganese (II)? duab 14
- coj (II)? duab 15
Cov txheej txheem ntawm cov txheej txheem tsis tu ncua yog raws li qhov tshwm sim ntawm osmosis, piv txwv li, nkag mus ntawm cov khoom me me los ntawm qhov pores ntawm semipermeable membranes. Deposits ntawm insoluble hlau silicates daim ntawv raws li ib tug nyias txheej ntawm cov ntsev nkag mus rau hauv lub xeem raj. Cov dej molecules nkag mus rau hauv lub qhov hws ntawm daim nyias nyias, ua rau cov hlau ntsev hauv qab kom yaj. Cov kev daws teeb meem thawb cov zaj duab xis kom txog thaum nws tawg. Tom qab pouring tawm cov hlau ntsev tov, puas silicate precipitate re-precipitate? lub voj voog rov ua nws tus kheej thiab cov tshuaj cog? nce.
Los ntawm kev muab cov ntsev ntsev ntawm ntau yam hlau tso rau hauv ib lub nkoj thiab tso dej nrog cov kua iav, peb puas tuaj yeem loj hlob tag nrho "chemical vaj"? (Duab 16, 17, 18).
Cov duab