Reactions ntawm mercury compounds
Hlau mercury thiab nws cov tebchaw muaj tshuaj lom heev rau cov kab mob nyob. Qhov no yog tshwj xeeb tshaj yog muaj tseeb rau cov tebchaw uas muaj soluble nyob rau hauv dej. Kev saib xyuas zoo yuav tsum tau ua thaum sim nrog kev sib xyaw ua ke ntawm cov khoom tshwj xeeb no (mercury tsuas yog cov hlau uas yog kua hauv chav sov). Ua raws li cov kev cai hauv paus ntawm tus kws tshuaj? yuav tso cai rau koj kom muaj kev nyab xeeb ua ntau yam kev sim nrog cov tshuaj mercury.
Hauv thawj qhov kev sim, peb tau txais aluminium amalgam (ib qho kev daws ntawm cov hlau no hauv cov kua mercury). Mercury (II) tov Hg nitrate (V) Hg (NO3)2 thiab ib daim ntawm txhuas hlau (duab 1). Ib qho txhuas pas nrig (ua tib zoo ntxuav ntawm qhov tso nyiaj) yog muab tso rau hauv lub raj ntsuas nrog cov kua ntsev mercury soluble (duab 2). Tom qab qee lub sijhawm, peb tuaj yeem soj ntsuam qhov tso pa roj npuas los ntawm qhov chaw ntawm cov hlau (duab 3 thiab 4). Tom qab tshem tus pas nrig los ntawm kev daws, nws hloov tawm tias cov av nplaum tau npog nrog cov txheej txheem fluffy, thiab ntxiv rau, peb kuj pom cov pob ntawm cov hlau mercury (duab 5 thiab 6).
Chemistry - qhov kev paub ntawm kev sib txuas mercury
Nyob rau hauv ib txwm muaj xwm txheej, qhov saum npoo ntawm txhuas yog coated nrog ib tug nruj haum txheej ntawm txhuas oxide.2O3zoo cais cov hlau los ntawm kev txhoj puab heev ib puag ncig influences. Tom qab ntxuav thiab immersing tus pas nrig nyob rau hauv ib qho kev daws ntawm mercury ntsev, Hg ions raug tshem tawm.2+ ntau active aluminium
Mercury tso rau ntawm qhov chaw ntawm tus pas nrig ua ib qho amalgam nrog txhuas, uas ua rau nws nyuaj rau oxide kom ua raws li nws. Aluminium yog ib yam hlau uas muaj zog heev (nws hnov mob nrog dej kom tso tawm hydrogen - cov pa npuas tau pom), thiab nws siv los ua cov khoom siv tau zoo vim yog cov txheej txheem oxide tuab.
Hauv kev sim thib ob, peb yuav kuaj ammonium NH ions.4+ siv Nessler's reagent (tus kws tshuaj German Julius Nessler yog thawj zaug siv nws hauv kev tshuaj xyuas xyoo 1856).
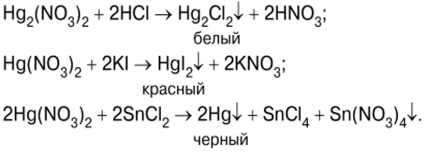
Kev sim ntawm cov tshuaj tiv thaiv ntawm hops thiab mercury compounds
Qhov kev sim pib nrog nag lossis daus ntawm mercury (II) iodide HgI.2Tom qab sib tov daws cov poov tshuaj iodide KI thiab mercury (II) nitrate (V) Hg (NO)3)2 (Duab 7):
Txiv kab ntxwv-liab precipitate ntawm HgI2 (Daim duab 8) tom qab ntawd kho nrog ntau tshaj ntawm cov tshuaj potassium iodide kom tau txais cov tshuaj soluble complex ntawm cov mis K2HgI4 ? Potassium tetraiodercurate (II) (Duab 9), uas yog Nessler's reagent:
Nrog rau qhov tshwm sim compound, peb tuaj yeem ntes ammonium ions. Kev daws teeb meem ntawm sodium hydroxide NaOH thiab ammonium chloride NH tseem yuav xav tau.4Cl (daim duab 10). Tom qab ntxiv ib qho me me ntawm ammonium ntsev tov rau Nessler reagent thiab alkalizing nruab nrab nrog lub hauv paus muaj zog, peb soj ntsuam qhov tsim ntawm cov xim daj-txiv kab ntxwv ntawm cov ntsiab lus ntawm cov raj. Cov tshuaj tiv thaiv tam sim no tuaj yeem sau ua:
Lub resulting mercury compound muaj ib tug complex qauv:
Qhov kev ntsuam xyuas Nessler rhiab heev yog siv los txheeb xyuas cov kab ntawm ammonium ntsev lossis ammonia hauv dej (xws li dej kais).

